Thermodynamic Process
Thermodynamic Process: Overview
This topic consists of various concepts like Non-cyclic process,Quasi-static Process,Isothermal Process and Its Equation of State, etc.
Important Questions on Thermodynamic Process
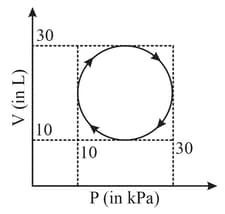
Heat absorbed by a system in going through a cyclic process shown in figure is :
What is the meaning of adiabatic expansion?
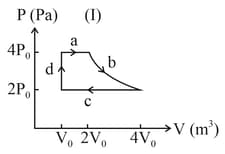
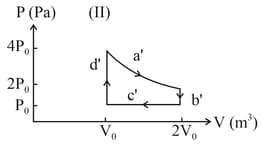
One mole of an ideal gas undergoes two different cyclic processes and , as shown in the diagrams below. In cycle , processes and are isobaric, isothermal, isobaric and isochoric, respectively. In cycle , processes and are isothermal, isochoric, isobaric and isochoric, respectively. The total work done during cycle is and that during cycle is . The ratio is _____.
A closed container contains a homogeneous mixture of two moles of an ideal monatomic gas and one mole of an ideal diatomic gas . Here, is the ratio of the specific heats at constant pressure and constant volume of an ideal gas. The gas mixture does a work of Joule when heated at constant pressure. The change in its internal energy is _____ Joule.
The initial pressure and volume of an ideal gas are and . The final pressure of the gas when the gas is suddenly compressed to volume will be:
(Given = ratio of specific heats at constant pressure and at constant volume.)
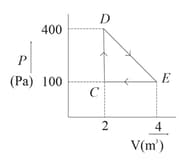
A thermodynamic system is taken through cyclic process. The total work done in the process is :
Consider two containers and containing monoatomic gases at the same Pressure , Volume and Temperature . The gas in is compressed isothermally to of its original volume while the gas in is compressed adiabatically to of its original volume. The ratio of final pressure of gas in to that of gas in is
A gas is compressed adiabatically, which one of the following statement is NOT true?
A mixture of gases with adiabatic coefficient equal to is compressed from initial state to one fourth volume adiabatically. Its final pressure will be equal to
A sound wave is travelling in a uniform pipe with gas of adiabatic exponent . If is the particle velocity at any point in medium and is the wave velocity, then relative change in pressure through this point is :-
An ideal gas is compressed such that its pressure and volume are related as = constant. During this process, the temperature of gas
In which of the following process, the internal energy of gas remains constant?
A monoatomic gas initially at pressure and volume is compressed to of its volume adiabatically. Final pressure of the gas is equal to
The final volume (in ) of one mole of an ideal gas initially at and pressure, if it absorbs of heat during a reversible isothermal expansion, is
The work done by gas is equal to heat supplied. An ideal gas has initial volume and pressure To triple its volume the minimum work done, will be
Following graph shows a single stage expansion process, then work done by the system is ?
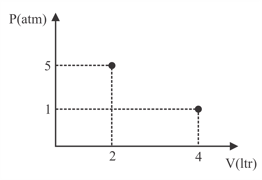
Select the correct option regarding adiabatic expansion of an ideal gas.
A closed gas cylinder is divided into two parts by a piston held tight. The pressure and volume of gas in two parts respectively are and . If now the piston is left free and the system undergoes isothermal process, then the volume of the gas in two parts respectively are
A monoatomic gas does of work when it is expanded isobarically. How much of heat is given to the gas in the process
A gas is expanded from an initial state to a final state along a path on a diagram. The path consists of (i) an isothermal expansion of work , (ii) an adiabatic expansion and (iii) an isothermal expansion of work . If the internal energy of gas is changed by , then the work done by gas during adiabatic expansion is
